
Plating is the process of plating a process of depositing a thin layer of another metal or alloy onto the surface of certain metals using the principle of electrolysis. It is a process of using electrolysis to attach a layer of metal film to the surface of a metal or other material to prevent metal oxidation ( Such as rust), improve wear resistance, conductivity, reflectivity , corrosion resistance, and enhance aesthetics.
During Plating, the coating metal or other insoluble materials are used as the positive pole, and the workpiece to be plated is used as the cathode. The cations of the coating metal are reduced on the surface of the workpiece to form a coating. In order to eliminate the interference of other cations and ensure that the coating is uniform and firm, it is necessary to use a solution containing metal cations as the Plating solution to keep the concentration of the metal cations in the coating constant. Figure 1 Plating Schematic Diagram.
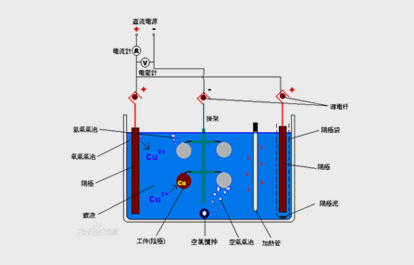
Plating mainly satisfies the main functions of decoration, functionality and corrosion resistance. Different Plating layers have different functions.
The plating layer is divided into single metal plating, alloy plating and composite plating.
Single metal Plating has a history of more than 170 years. There are already 33 kinds of metals on the periodic table that can be deposited from an aqueous solution. There are more than 10 kinds of Plating zinc, nickel, chromium, copper, tin, iron, cobalt, cadmium, lead, gold, silver, etc.
The coating formed by depositing two or more than two elements simultaneously on the cathode is an alloy coating. The alloy coating has the structure and properties that a single metal coating does not have, such as amorphous Ni-P alloy, sn alloys of various cores that are not on the phase diagram, and has a special decorative appearance, particularly high corrosion resistance and excellent weldability , Magnetic alloy coating, etc.
Composite coatings currently mainly refer to multi-layer coatings, which are mainly corrosion-resistant in application, such as long-term salt spray, artificial sweat test, and electrolytic test.
With the development of the market, more and more customers have higher and higher requirements for product quality. In order to meet the needs of customers, HYP has invested a lot of personnel, machinery and equipment, and the latest technology to develop a variety of special Plating products lines, from traditional nickel-plated, gold-plated coatings to special sweat-resistant, electrolytic-resistant coatings.
Related news
-

Common items and methods for precision metal plating testing
Electroplatingisacommonmetalsurfacetreatmentsolution.Electroplatingcannotonlyincreasetheappearanceoftheproduct,butalsoimprovethepe... -

Key points of electroplating of pogo pin parts in precision electronic connector electroplating
Pogopin is a common electronic connector. Due to its size and precision, it is generally processed by roll plating. The following is the key points of electroplating from the three parts of pogopin: spring, barrel and plunger: -

Advantages and disadvantages of common electroplating layers in medical microneedle electroplating
Due to the wide application and particularity of medical microneedles, the electroplating layer of medical microneedles plays an important role in their application. The following introduces the common electroplating layers of medical microneedle electroplating processing. -

Environmental nickel free plating manufacturer - copper tin zinc plating
One in every 1 million people in the world is allergic to metals. The most common allergen metal is nickel. Once allergies occur, it can cause contact dermatitis manifestations such as redness, itching, and yellow water around the contact. These are all adverse reactions caused by the release of metal ions. Once metal allergic dermatitis occurs, the most fundamental and effective treatment The solution is to isolate the allergen. -

Wastewater Treatment at HYP
Electroplating enterprises and surface treatment enterprises have chemical oxidation, anodic oxidation, galvanizing, zinc-nickel alloy plating, chrome plating, copper plating, gold plating, nickel plating, silver plating and other plating types, and the wastewater produced is very messy.





